Since 1968, Gull Industries is the premier metal coating service provider throughout the central, southern region of North America. We pride ourselves as being a customer and market focused leader relative to customer services, product quality and environmental leadership. We apply 'Hi-Phos' E-Nickel at Gull Industries which is the most corrosion resistant version of all ENP processes available.

Cal-Aurum Industries provides metal plating and metal finishing systems to a variety of industries. We offer electroless nickel plating as well as gold, silver, rhodium, palladium, nickel, tin, tin/lead and copper plating. Our company formed in 1971, so we have years of experience and expertise to offer when we hear form you!

Since 1975, AOTCO Metal Finishing has been specializing in metal finishing systems and metal plating including tin, gold, copper, cadmium, chromium and electroless nickel plating. We are a metal finisher where quality and reliability count the most. Call us for more information.

More Tin Plating Companies
Some of these beneficial characteristics of tin, particularly as a plating material, include its non-toxicity, high ductility and high corrosion-resistance.
The two most common materials that tin is used as a protective coating for include nickel and copper; however, tin plating cannot be used as a protective coating for steel. Utilized in a variety of applications, tin plating is an essential process in industries such as: power generation, for plating of machinery such as power sub-stations, high-voltage connections and power grounding; electronics, to increase conductivity of electronic devices such as capacitors and printed circuit boards (PCBs); industrial manufacturing, for plating of parts requiring protection from harsh environmental factors; and food processing, in which tin plating is especially useful because it is not a toxic metal. Tin is typically alloyed with other metals such as lead or copper before it is used for electroless plating in order to prevent tiny crystalline structures from occurring on the surface of the plated substance.
The typical electroless plating process for tin includes immersion of the tin into an aqueous bath, in which several chemical reactions occur in order to enable the nickel to deposit a thin layer of material onto the desired ferrous or nonferrous metal workpiece. Several chemical reactions occur because of the introduction of both a reactive agent and an accelerant. The reactive agent enables the deposition, while the accelerant removes any remnants of the reactive agent from the workpiece.
The electroless method of tin plating is very different from the electrolytic method of tin plating. In the electrolytic method of tin plating, an electrical current is used to reduce the number of cations of tin from a solution. To begin the process, the part to be plated is considered to be the cathode of the circuit and, in certain methods, the anode is made of tin.
Both the metal to be plated and the tin are immersed in an aqueous solution called an electrolyte. This solution contains one or more dissolved metallic salts in addition to other ions that permit electrical flow. A rectifier supplies a direct current (DC) to the anode, allows oxidation of the tin to occur and then dissolve in the solution. At the cathode, the dissolved tin ions are reduced in the solution between the solution and the cathode, thus causing deposition of a thin layer of tin onto the desired material to occur.





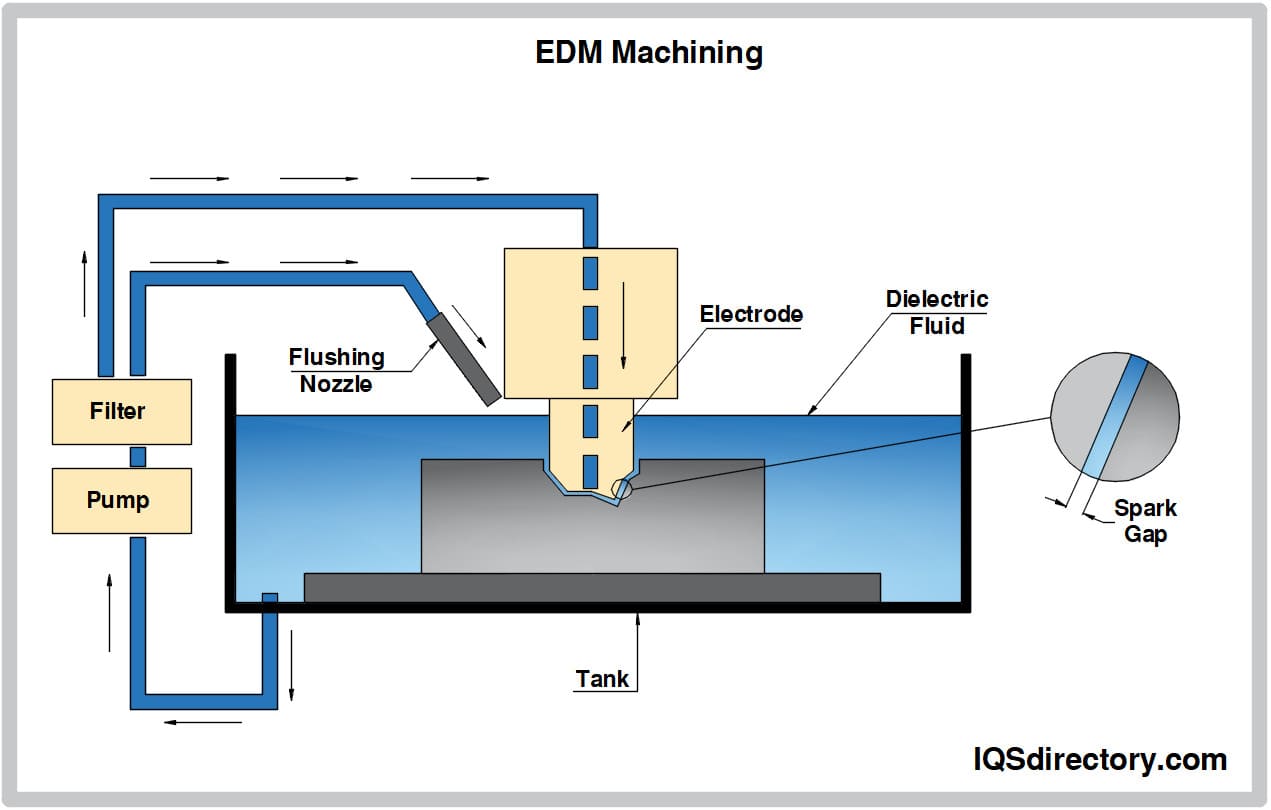
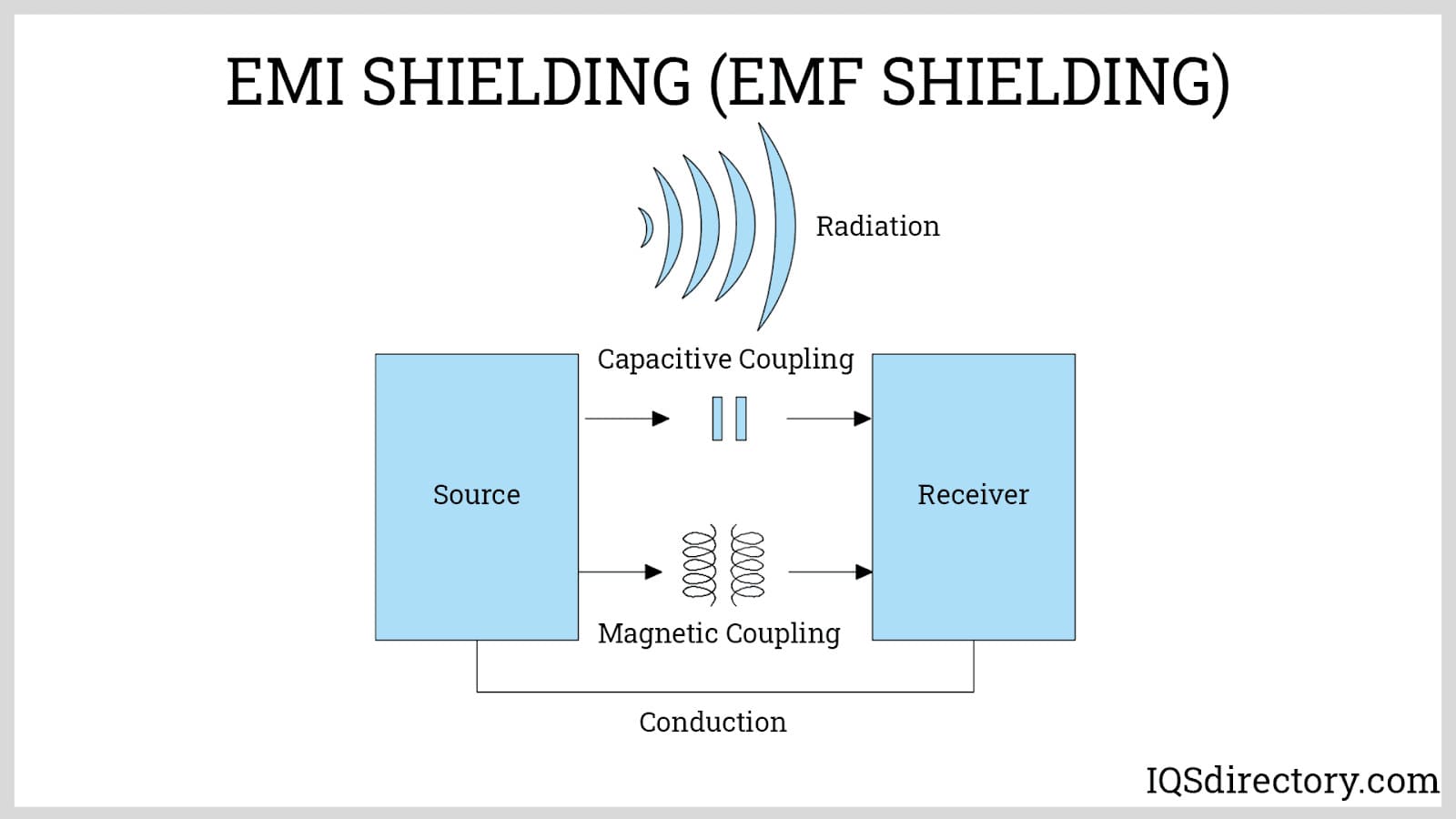
 Aluminum Anodizing
Aluminum Anodizing EDM
EDM Electroless Nickel Plating
Electroless Nickel Plating EMI Shielding
EMI Shielding Heat Treating
Heat Treating Metal Coating Services
Metal Coating Services Castings & Forgings
Castings & Forgings Bulk Material Handling
Bulk Material Handling Electrical & Electronic Components
Electrical & Electronic Components Flow Instrumentation
Flow Instrumentation Hardware
Hardware Material Handling Equipment
Material Handling Equipment Metal Cutting Services
Metal Cutting Services Metal Forming Services
Metal Forming Services Metal Suppliers
Metal Suppliers Motion Control Products
Motion Control Products Plant & Facility Equipment
Plant & Facility Equipment Plant & Facility Supplies
Plant & Facility Supplies Plastic Molding Processes
Plastic Molding Processes Pumps & Valves
Pumps & Valves Recycling Equipment
Recycling Equipment Rubber Products & Services
Rubber Products & Services