Since 1968, Gull Industries is the premier metal coating service provider throughout the central, southern region of North America. We pride ourselves as being a customer and market focused leader relative to customer services, product quality and environmental leadership. We apply 'Hi-Phos' E-Nickel at Gull Industries which is the most corrosion resistant version of all ENP processes available.

Cal-Aurum Industries provides metal plating and metal finishing systems to a variety of industries. We offer electroless nickel plating as well as gold, silver, rhodium, palladium, nickel, tin, tin/lead and copper plating. Our company formed in 1971, so we have years of experience and expertise to offer when we hear form you!

Since 1975, AOTCO Metal Finishing has been specializing in metal finishing systems and metal plating including tin, gold, copper, cadmium, chromium and electroless nickel plating. We are a metal finisher where quality and reliability count the most. Call us for more information.

More Electroless Nickel Plating Companies
Electroless nickel plating is a process that uses auto-catalytic chemical reactions to create a layer of nickel alloy to coat a metal or plastic object. The process works in such a way that the nickel alloy coating deposits directly onto the substrate as the chemical reaction creates it.
A similar process, electroplating, plates metal by making use of an electrical current that allows for metal plating with electricity. Electroless nickel plating, on the other hand, uses no electricity.
Electroless nickel plating has many other names, including electroless nickel coating, EN plating, E/Ni plating, chemical nickel plating, autocatalytic nickel plating, autocatalytic plating, and autocatalytic coating. More broadly, manufacturers refer to this process as electroless plating. This is because, while nickel is most common, they can perform this process using other metals.
Applications
Electroless nickel plating is a service that manufacturers very commonly perform on products used in industrial applications. They optimize it for industries like chemical, petroleum, automotive, aerospace, electronics, military and defense, household, food preparation, general manufacturing, jewelry, and more.
Electroless nickel plating is a popular service because nickel coatings provide a wide range of advantages and protections to their applications. Such advantages include durability, corrosion protection/corrosion resistance, abrasion resistance, wear resistance, electromagnetic shielding (electronics), hardness, chemical resistivity (aerospace), lubricity (aerospace), cleanliness, and attractive metal finishing.
Products Treated
Using electroless nickel plating, manufacturers can treat all sorts of products. Check out the wide range of examples below, which we have organized by industry.
- Chemical: turbine blades, pressure vessels, hydraulic components
- Petroleum: fuel rails, oilfield valves
- Automotive: brake pistons, drive shafts, mufflers, rotors, power transmission
- Aerospace: propellers, engine shafts, engine mounts, compressor blades, land gear equipment
- Electronics: connectors, hard drive disks, wire terminals, printed circuit boards (PCBs)
- Military and defense: firearms and other weapons, mirrors, fuse assemblies
- Household: bathroom fixtures, door knobs, door and cabinet fasteners, stainless steel utensils
- Food prep: grills, molds, canning machines, other food service equipment
- Manufacturing: pipes, tools, gears, fasteners, etc.
- Jewelry: diamond turning, other optical surfaces
History
Electroless nickel plating was not invented so much as stumbled upon. Between 1841 and 1845, Adolphe Wurtz, an Alsatian French chemist, published two papers discussing hypophosphorous acid and other phosphorous acids. While he studied these acids, he unwittingly discovered that when immersed in a chemical reduction bath, nickel-phosphorous will deposit its ion on a present surface.
This was not Wurtz’s primary scientific interest, so he moved on quickly. Others, however, found it intriguing and sometime after his discovery, they picked up electroless nickel baths to study. In 1916, F.A. Roux, another chemist, was the first to take out an American patent on electroless nickel coating. In his patent, he described the nickel depositing process as “spontaneous and complete.”
Despite the efforts of Roux, electroless nickel plating remained obscure until the 1940s. In 1946, Grace Riddell, along with Abner Brenner, further developed electroless nickel plating for the purpose of depositing nickel-tungsten onto the inner walls of tubes. Riddell and Brenner received a patent for this nickel deposition process in 1950, with a few amendments so that people were aware that it was different from the one patented by Roux. Originally, Riddell and Brenner called electroless plating “electrodeless plating.” Before patenting it, they changed it.
Until 1963, the electroless nickel plating process was classified, as manufacturers had used it during World War II on military equipment. After the U.S. government declassified it in 1963, civilian engineers and manufacturers began using the process for their applications. Also, as the years went on, chemists and engineers learned how to use the process to deposit other types of materials and alloys, like diamond and even PTFE. For example, in 1974, K. Parker tested electroless plating composite procedures that included the deposition of coatings of Teflon, boron carbide (nickel-boron coatings), titanium carbide, chromium carbide, silicon carbide, tungsten carbide, occluded graphite, and aluminum oxide.
Today electroless nickel plating is an incredibly important process, especially in electronics. As time goes on, we can expect this importance to grow even more. Manufacturers can now deposit coatings of anything from copper and zinc deposits to palladium deposits.
Materials
In addition to nickel, materials manufacturers can use in an electroless plating process include silver, gold, tin, copper, zinc, chrome, cadmium, palladium, and rhodium. The most popular of these nickel deposit alternatives are gold, copper, silver, and palladium.
Electroplating (electrically charged) processes use a wider variety of metals; from tin to zinc to chrome to cadmium and rhodium.
Gold plated objects are given a thin layer of gold across the surface of a different metal. The purpose of depositing gold is usually to provide electronics with a layer that is both corrosion resistant and conductive.
Tin adds conductivity when combined with other metals like copper before plating takes place.
Zinc is used to prevent oxidation of the plated metal. Not only that, but zinc is used in processes where small parts are plated together in large groups.
Hard chrome plating leaves a polished finish, but it can be expensive and it requires more electrical current than other metals.
Silver plating can be found in electronics as well and makes a cheaper alternative to gold. The main problem with silver is that it does not oxidize so it will not perform well in humid environments.
Copper works well for many applications. This is mainly because of copper’s high conductivity. People also like copper for its color.
Palladium offers unmatched bath stability, but it is rare and therefore quite expensive to source.
Cadmium plating (electroless) is possible, but it is not common because the metal can be a hazard to the environment. This has made it very controversial among manufacturers.
Rhodium plating (electroless) is used on precious metals, particularly for jewelry.
Also, manufacturers may conduct electroless nickel plating with nickel-phosphorus alloys containing three different general concentrations of phosphorus. Nickel-phosphorus alloys with these concentrations can be divided into three groups: low phosphorus alloys, medium phosphorus alloys, and high phosphorus alloys.
Low phosphorus nickel-phosphorus plating materials use less than 5% phosphorus. Featuring a crystalline nickel deposit structure, of all combinations, they have the best solderability, highest initial surface hardness (surface hardness without post-plating heat treatment) at up to 60 RC (Rockwell hardness scale measurement), lowest electrical resistance, and best resistance to alkaline environments.
Medium phosphorus nickel-phosphorus plating materials have between 5% and 9% phosphorus content. Of all the nickel-phosphorus combinations, they create the most aesthetically pleasing plating. They deposit a crystalline or mixed amorphous structure.
High phosphorus nickel-phosphorus plating materials are composed of between 10% and 13% phosphorus. With this amount of phosphorus content present, they present the best ability to resist acidic environments and an above average ability to resist corrosion in general. When treated with post-plate thermal processing, high phosphorus nickel plating exhibits a hardness above 65 RC. The high corrosion resistance ability of this plating type is due in part to the amorphous structure of its deposit; the more amorphous the structure, the higher the corrosion protection.
Process Details
Electroless nickel plating is a widely used process for depositing a layer of nickel onto a substrate without the need for an external electric current. The process typically involves several key steps:
Surface Preparation
The first step in electroless nickel plating is surface preparation. The substrate that needs to be plated is thoroughly cleaned to remove any dirt, grease, or contaminants from the surface. This is crucial to ensure proper adhesion between the substrate and the nickel coating. Surface preparation may involve processes such as degreasing, acid cleaning, or alkaline cleaning.
Activation
Once the surface is clean, it undergoes activation to create nucleation sites for the nickel deposition. The activation step is essential because it allows the subsequent plating solution to adhere properly to the substrate. There are various methods of activation, with the most common involving the use of acidic solutions or sensitizing agents like palladium chloride. The activation process often involves immersing the substrate in the activation solution for a specific period.
Plating Bath Preparation
The plating bath is a carefully formulated solution that contains nickel ions, reducing agents, stabilizers, complexors, and other chemicals. The nickel source is usually nickel sulfate or nickel chloride. The reducing agent is a crucial component, as it is responsible for reducing the nickel ions to deposit metallic nickel on the activated surface. Sodium hypophosphite is the most commonly used reducing agent, but other options like borohydride-based solutions are also employed for specific applications.
Immersion in the Plating Bath
After activation, the substrate is immersed in the plating bath. Unlike traditional electroplating, electroless nickel plating does not require an external electric current to drive the deposition process. Instead, the nickel ions are reduced directly on the catalytic surface of the substrate. The reduction reaction takes place on the activated sites, and the nickel atoms are deposited as a thin metallic layer on the substrate surface.
Autocatalytic Deposition
The plating process is autocatalytic, meaning that the deposited nickel layer itself acts as a catalyst for the ongoing reduction reaction. As the nickel layer grows, it continues to catalyze the reduction of nickel ions in the plating bath, resulting in a self-sustaining plating process until the desired coating thickness is achieved.
Controlling Plating Parameters
The plating process is closely monitored and controlled to ensure a consistent and uniform coating. Factors like bath temperature, pH level, concentration of nickel ions, and reducing agent are carefully maintained within specific ranges to achieve the desired plating rate and thickness.
Rinsing and Drying
Once the desired thickness is reached, the plated substrate is removed from the plating bath, rinsed thoroughly to remove any residual chemicals, and then dried.
The resulting electroless nickel coating provides several advantages, including excellent corrosion resistance, wear resistance, and hardness. It finds applications in various industries such as automotive, aerospace, electronics, and chemical manufacturing, where components require protection from environmental factors and improved surface properties.
Note: Manufacturers often also use hydrated sodium hypophosphite or similar reducing agents in this process. This is because they react directly with the metal ions and allow the nickel to be deposited on the surface.
Considerations
Before initiating electroless nickel plating, manufacturers carefully consider several factors. First, they evaluate the substrate material’s compatibility with the plating process, as different materials may require specific surface preparation or activation techniques to achieve proper adhesion. Thorough surface cleaning and preparation are essential to remove contaminants and ensure a clean substrate for plating. The choice of activation method is critical, as it influences nucleation sites and the adhesion of the subsequent nickel layer.
During the electroless nickel plating process, meanwhile, manufacturers pay close attention to various parameters. They continuously monitor and control the plating bath conditions, including temperature, pH, and chemical concentrations, to maintain optimal levels for achieving the desired plating rate and uniform coating thickness. Adequate agitation of the plating bath ensures a consistent distribution of chemicals and prevents localized variations in the coating. The plating time is carefully regulated to strike a balance between achieving the required thickness and avoiding over-plating, which could lead to increased costs or quality issues.
After the electroless nickel plating process, several additional considerations come into play. Manufacturers may choose to subject the plated components to post-treatment processes based on the application requirements. Post-treatment methods may include heat treatment for improved hardness or stress relief, passivation to enhance corrosion resistance, or other specific treatments to tailor the coating’s properties. Thorough inspection and quality control measures are typically carried out to ensure the coating adheres uniformly and meets required specifications. This involves conducting thickness measurements, adhesion testing, and surface analysis. Finally, proper packaging and storage factors are crucial to protect the plated components from damage during transportation and maintain the integrity of the nickel coating until the components are ready for use. By addressing these considerations, manufacturers can produce high-quality electroless nickel coatings that meet the specific performance and durability needs of their intended applications.
Advantages over Similar Processes
Electroplating is a process that involves the deposition of a metal coating onto a substrate using an external electric current. Unlike electroless nickel plating, which does not require an external current, electroplating relies on the electrolysis principle. The substrate is immersed in an electrolyte solution containing metal ions, and an electric current is applied to attract and deposit the metal ions onto the substrate.
Electrochemical deposition is another process that resembles electroless nickel plating. It involves the electrodeposition of metals from an electrolyte solution onto a conductive substrate. However, electrochemical deposition also requires the application of an external electric current to facilitate the metal ion transfer from the electrolyte to the substrate surface. This process is more common for plating specific metals like copper or gold rather than nickel.
Electroless nickel plating offers several distinct advantages over electroplating and electrochemical deposition processes. To start, electroless nickel plating provides exceptional coating uniformity and conformity, even on complex and irregularly shaped components, ensuring a seamless and continuous coating without the risk of uneven thickness or voids often encountered in electroplating and electrochemical deposition. Another key advantage is that electroless nickel plating does not require an external electric current, simplifying the plating setup and eliminating the need for precise current density control, reducing the potential for defects like burn marks. Moreover, the autocatalytic nature of electroless nickel plating results in better adhesion to the substrate surface, forming strong bonds that offer superior wear resistance and corrosion protection. Additionally, the versatility of electroless nickel plating, with options like high-phosphorus, medium-phosphorus, and low-phosphorus nickel solutions, allows manufacturers to tailor the coating’s properties to specific application requirements. Finally, electroless nickel plating is generally more cost-effective and simpler in terms of setup and equipment compared to electroplating and electrochemical deposition processes. These advantages make electroless nickel plating the preferred choice for various applications that demand high-quality, uniform, and reliable metal coatings.
Things to Consider
If you’re in the market for electroless nickel plating services, your best bet is to connect with service providers that are backed up by satisfied customers and a high-quality body of work. Of course, when you’re looking on the internet, it’s easy to get overwhelmed by the endless results a Google search will give you. To help you narrow down your search to a small group of high quality candidates, we’ve listed some of those we trust most.
Before you start browsing, we recommend you take some time to put together a list of your own. This list should be a compilation of all your application specifications and requirements. Don’t forget to write down your standard requirements, your budget, your delivery preferences, and your timeline. Once you have written down your specifications, take a look at the profiles of those suppliers we have listed on this page. Use your list as a guide for the quick sorting of these electroless nickel plating providers into yes and no groups. Pick three or four manufacturers that you believe may best serve you and reach out to each of them. After you have discussed your application with each potential plating supplier, compare and contrast them, and pick the one for you. Good luck!



 Aluminum Anodizing
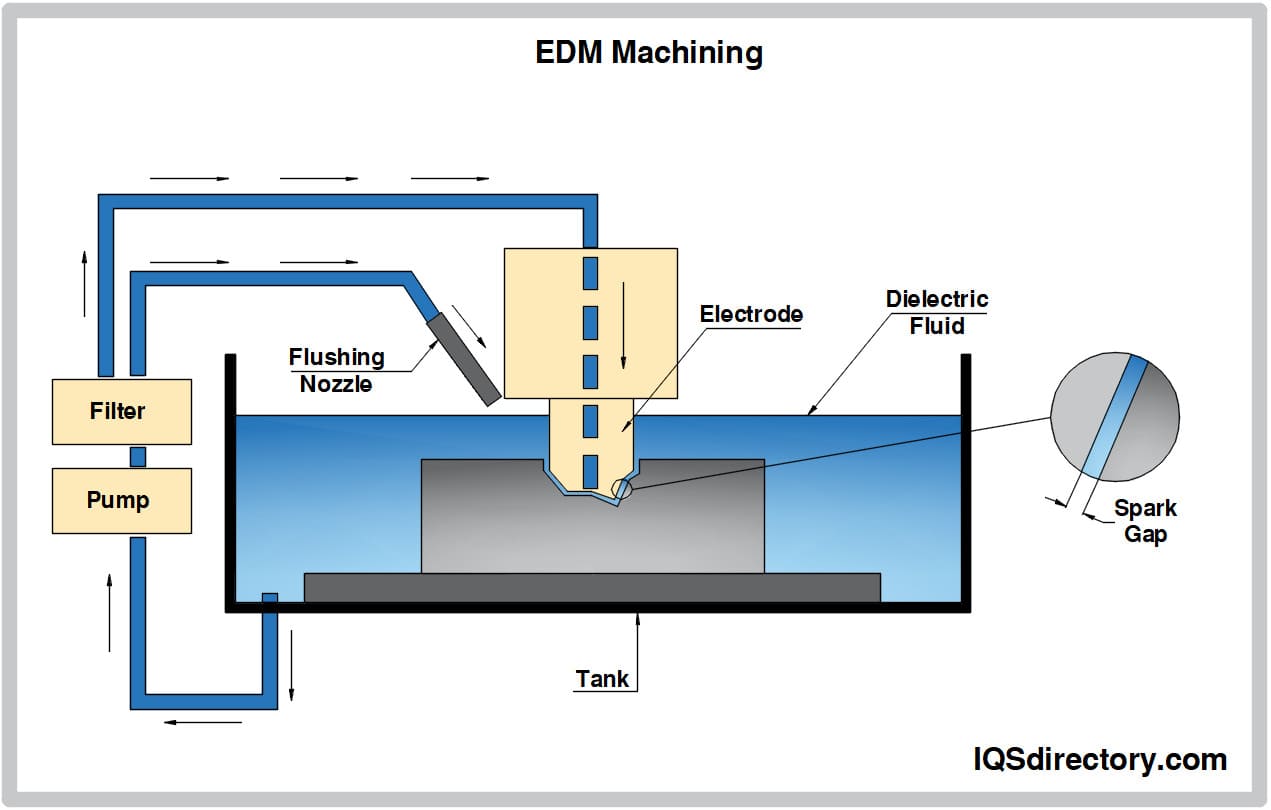
Aluminum Anodizing EDM
EDM Electroless Nickel Plating
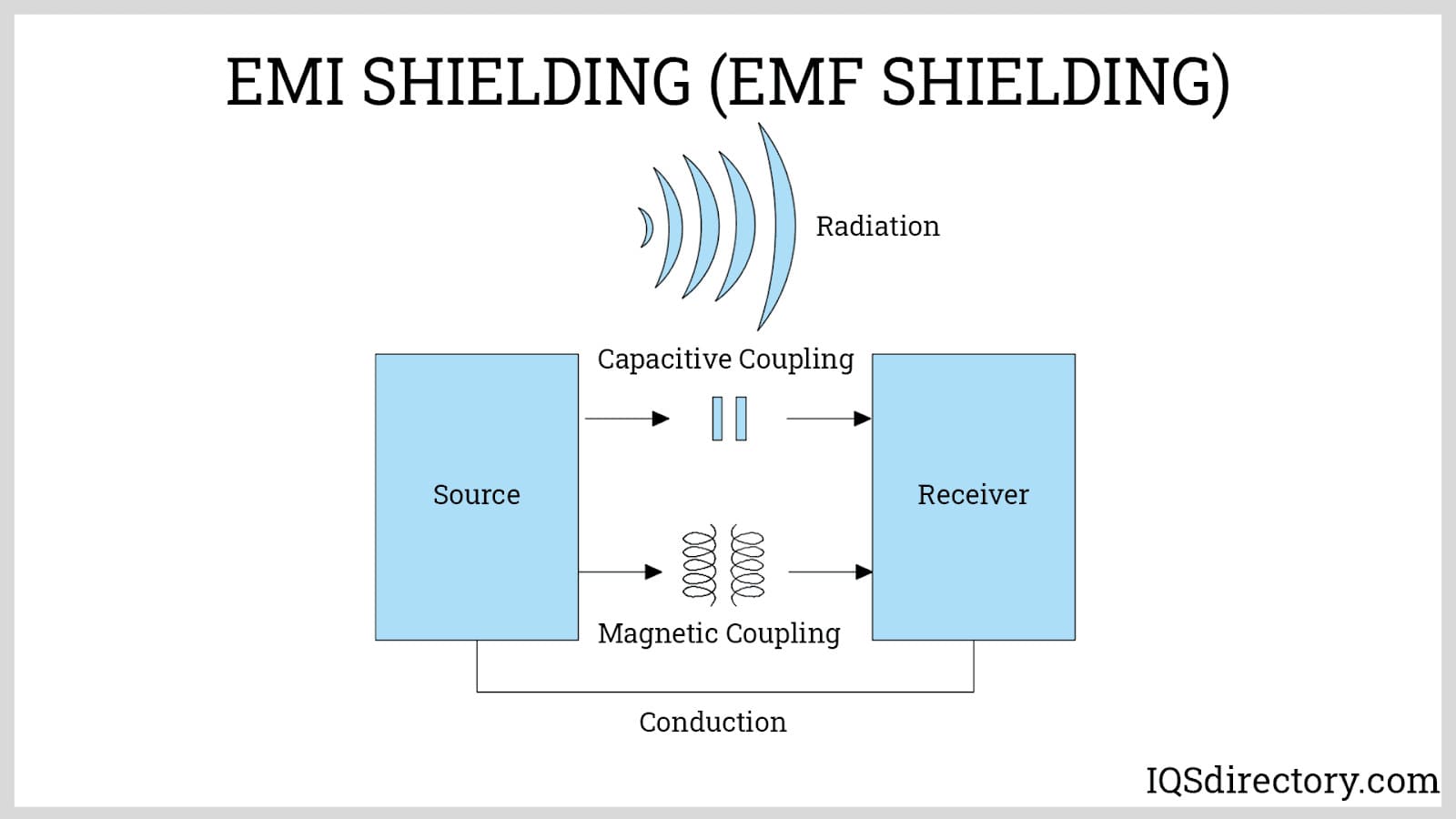
Electroless Nickel Plating EMI Shielding
EMI Shielding Heat Treating
Heat Treating Metal Coating Services
Metal Coating Services Castings & Forgings
Castings & Forgings Bulk Material Handling
Bulk Material Handling Electrical & Electronic Components
Electrical & Electronic Components Flow Instrumentation
Flow Instrumentation Hardware
Hardware Material Handling Equipment
Material Handling Equipment Metal Cutting Services
Metal Cutting Services Metal Forming Services
Metal Forming Services Metal Suppliers
Metal Suppliers Motion Control Products
Motion Control Products Plant & Facility Equipment
Plant & Facility Equipment Plant & Facility Supplies
Plant & Facility Supplies Plastic Molding Processes
Plastic Molding Processes Pumps & Valves
Pumps & Valves Recycling Equipment
Recycling Equipment Rubber Products & Services
Rubber Products & Services